Unlock the Power of
Gastric-Intelligence
Over 24 Million Enteral Feeding tubes are placed each year. We’ve made them smart. Deliver safe, simplified enteral care faster. Identify life-threatening complications sooner. Save lives.

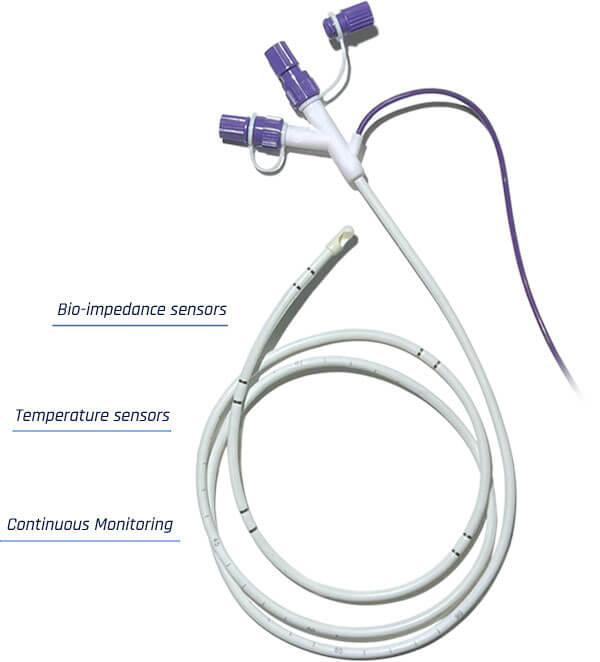
Introducing Entarik:
An Enteral Nutrition
Intelligence Platform
Entarik utilizes embedded sensors to collect over 140 “gut data” samples per second.
Safe Enteral Feeding
Confidently place NG Tubes while reducing fear of injuring patients.
Clinical Evidence:
Clinical study reveals 100% accuracy of Nasogastric Tube Placement using Entarik as the guide.


Future Innovation
Entarik is designed to scale. Capture and analysis of GI data may be useful for early identification of ICU patient complications.

